POMALYST Indication
POMALYST® (pomalidomide) is a thalidomide analogue indicated for the treatment of adult patients:
- in combination with dexamethasone, for patients with multiple myeloma who have received at least two prior therapies including lenalidomide and a proteasome inhibitor and have demonstrated disease progression on or within 60 days of completion of the last therapy.
POMALYST + dexamethasone + daratumumab Indication (DPd)
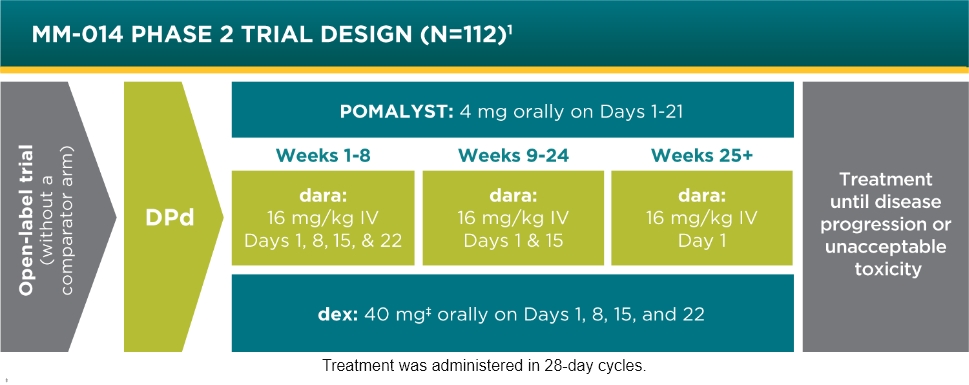
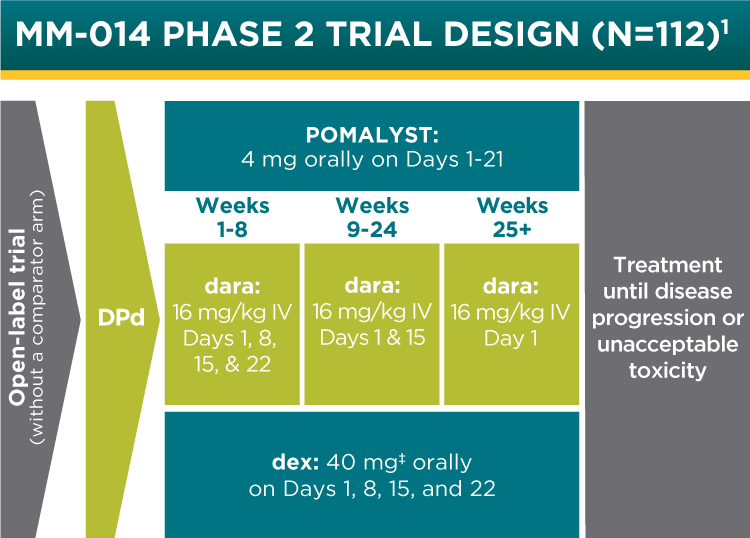
POMALYST + dexamethasone + daratumumab is indicated for the treatment of adult patients with multiple myeloma who have received at least two prior therapies including lenalidomide and a proteasome inhibitor.
POMALYST + dexamethasone + daratumumab and hyaluronidase-fihj injection for subcutaneous use (dara SC) Indication (Pd + dara SC)
POMALYST + dexamethasone + daratumumab and hyaluronidase-fihj is indicated for the treatment of adult patients with multiple myeloma who have received at least one prior line of therapy including lenalidomide and a proteasome inhibitor.
Limitations of Use:
Daratumumab and hyaluronidase-fihj is not indicated and is not recommended for the treatment of patients with light chain (AL) amyloidosis who have NYHA Class IIIB or Class IV cardiac disease or Mayo Stage IIIB outside of controlled clinical trials.
Information about DPd and Pd + dara SC does not appear in the POMALYST Prescribing Information (PI). Please see the daratumumab and dara SC full PIs for a complete discussion of Important Safety Information at www.darzalexhcp.com/iv and www.darzalexhcp.com/faspro, respectively.
Important Safety Information for POMALYST, daratumumab, and dara SC
POMALYST Boxed WARNINGS
WARNING: EMBRYO-FETAL TOXICITY and VENOUS AND ARTERIAL THROMBOEMBOLISM
Embryo-Fetal Toxicity
- POMALYST is contraindicated in pregnancy. POMALYST is a thalidomide analogue. Thalidomide is a known human teratogen that causes severe birth defects or embryo-fetal death. In females of reproductive potential, obtain 2 negative pregnancy tests before starting POMALYST treatment.
- Females of reproductive potential must use 2 forms of contraception or continuously abstain from heterosexual sex during and for 4 weeks after stopping POMALYST treatment.
POMALYST is only available through a restricted distribution program called POMALYST REMS®.
Venous and Arterial Thromboembolism
- Deep venous thrombosis (DVT), pulmonary embolism (PE), myocardial infarction, and stroke occur in patients with multiple myeloma treated with POMALYST. Prophylactic antithrombotic measures were employed in clinical trials. Thromboprophylaxis is recommended, and the choice of regimen should be based on assessment of the patient’s underlying risk factors.
CONTRAINDICATIONS FOR POMALYST
- Pregnancy: POMALYST can cause fetal harm and is contraindicated in females who are pregnant. If the patient becomes pregnant while taking this drug, the patient should be apprised of the potential risk to a fetus.
- Hypersensitivity: POMALYST is contraindicated in patients who have demonstrated severe hypersensitivity (e.g., angioedema, anaphylaxis) to pomalidomide or any of the excipients.
CONTRAINDICATIONS FOR DARATUMUMAB
- Daratumumab is contraindicated in patients with a history of severe hypersensitivity (e.g., anaphylactic reactions) to daratumumab or any of the components of the formulation.
CONTRAINDICATIONS FOR DARA SC
- Daratumumab and hyaluronidase-fihj is contraindicated in patients with a history of severe hypersensitivity (e.g., anaphylactic reactions) to daratumumab, hyaluronidase, or any of the components of the formulation.
WARNINGS AND PRECAUTIONS FOR POMALYST
- Embryo-Fetal Toxicity & Females of Reproductive Potential: See Boxed WARNINGS for POMALYST
- Males: Pomalidomide is present in the semen of patients receiving the drug. Males must always use a latex or synthetic condom during any sexual contact with females of reproductive potential while taking POMALYST and for up to 4 weeks after discontinuing POMALYST, even if they have undergone a successful vasectomy. Males must not donate sperm.
- Blood Donation: Patients must not donate blood during treatment with POMALYST and for 4 weeks following discontinuation of POMALYST therapy because the blood might be given to a pregnant female patient whose fetus must not be exposed to POMALYST.
- POMALYST REMS Program: See Boxed WARNINGS
- Prescribers and pharmacies must be certified with the POMALYST REMS program by enrolling and complying with the REMS requirements; pharmacies must only dispense to patients who are authorized to receive POMALYST. Patients must sign a Patient-Physician Agreement Form and comply with REMS requirements; female patients of reproductive potential who are not pregnant must comply with the pregnancy testing and contraception requirements and males must comply with contraception requirements.
- Further information about the POMALYST REMS program is available at www.pomalystrems.com or by telephone at 1-888-423-5436.
- Venous and Arterial Thromboembolism: See Boxed WARNINGS for POMALYST. Patients with known risk factors, including prior thrombosis, may be at greater risk, and actions should be taken to try to minimize all modifiable factors (e.g., hyperlipidemia, hypertension, smoking). Thromboprophylaxis is recommended, and the choice of regimen should be based on assessment of the patient’s underlying risk factors.
- Increased Mortality With Pembrolizumab: In clinical trials in patients with multiple myeloma, the addition of pembrolizumab to a thalidomide analogue plus dexamethasone resulted in increased mortality. Treatment of patients with multiple myeloma with a PD-1 or PD-L1 blocking antibody in combination with a thalidomide analogue plus dexamethasone is not recommended outside of controlled clinical trials.
- Hematologic Toxicity: Neutropenia (46%) was the most frequently reported Grade 3 or 4 adverse reaction, followed by anemia and thrombocytopenia. Monitor complete blood counts weekly for the first 8 weeks and monthly thereafter. Patients may require dose interruption and/or modification.
- Hepatotoxicity: Hepatic failure, including fatal cases, has occurred in patients treated with POMALYST. Elevated levels of alanine aminotransferase and bilirubin have also been observed in patients treated with POMALYST. Monitor liver function tests monthly. Stop POMALYST upon elevation of liver enzymes. After return to baseline values, treatment at a lower dose may be considered.
- Severe Cutaneous Reactions: Severe cutaneous reactions including Stevens-Johnson syndrome (SJS), toxic epidermal necrolysis (TEN), and drug reaction with eosinophilia and systemic symptoms (DRESS) have been reported with POMALYST. DRESS may present with a cutaneous reaction (such as rash or exfoliative dermatitis), eosinophilia, fever, and/or lymphadenopathy with systemic complications such as hepatitis, nephritis, pneumonitis, myocarditis, and/or pericarditis. These reactions can be fatal. Consider POMALYST interruption or discontinuation for Grade 2 or 3 skin rash. Permanently discontinue POMALYST for Grade 4 rash, exfoliative or bullous rash, or any other severe cutaneous reactions such as SJS, TEN or DRESS.
- Dizziness and Confusional State: In patients taking POMALYST in clinical trials, 14% experienced dizziness (1% Grade 3 or 4) and 7% a confusional state (3% Grade 3 or 4). Instruct patients to avoid situations where dizziness or confusional state may be a problem and not to take other medications that may cause dizziness or confusional state without adequate medical advice.
- Neuropathy: In patients taking POMALYST in clinical trials, 18% experienced neuropathy (2% Grade 3 in one trial) and 12% peripheral neuropathy.
- Second Primary Malignancies (SPMs):
- Cases of acute myelogenous leukemia have been reported in patients receiving POMALYST as an investigational therapy outside of multiple myeloma.
- Monitor patients for the development of SPMs.
- Tumor Lysis Syndrome (TLS): TLS may occur in patients treated with POMALYST. Patients at risk are those with high tumor burden prior to treatment. These patients should be monitored closely and appropriate precautions taken.
- Hypersensitivity: Hypersensitivity, including angioedema, anaphylaxis, and anaphylactic reactions to POMALYST have been reported. Permanently discontinue POMALYST for angioedema or anaphylaxis.
WARNINGS AND PRECAUTIONS FOR DARATUMUMAB
- Infusion-Related Reactions: Daratumumab can cause severe and/or serious infusion-related reactions including anaphylactic reactions. These reactions can be life-threatening and fatal outcomes have been reported. Interrupt daratumumab infusion for infusion-related reactions of any severity. Permanently discontinue the infusion in case of anaphylactic reactions or life-threatening infusion reactions and institute appropriate emergency care.
- Interference With Cross-Matching and Red Blood Cell Antibody Screening: Daratumumab binds to CD38 on red blood cells (RBCs) and results in a positive Indirect Antiglobulin Test (Indirect Coombs test), which may persist for up to 6 months after the last daratumumab infusion. Type and screen patients prior to starting treatment. Inform blood banks that a patient has received daratumumab.
- Neutropenia: Monitor complete blood cell counts periodically during treatment. Monitor patients with neutropenia for signs of infection. Consider withholding daratumumab until recovery of neutrophils.
- Thrombocytopenia: Monitor complete blood cell counts periodically during treatment. Consider withholding daratumumab until recovery of platelets.
- Interference With Determination of Complete Response: Daratumumab can interfere with the determination of complete response and of disease progression in some patients with IgG kappa myeloma protein.
- Embryo-Fetal Toxicity: Can cause fetal harm. Advise pregnant women of the potential risk to a fetus and advise females of reproductive potential to use effective contraception.
WARNINGS AND PRECAUTIONS FOR DARA SC
- Hypersensitivity and Other Administration Reactions: Daratumumab and hyaluronidase-fihj can cause systemic administration-related reactions, including severe or life-threatening reactions, and local injection-site reactions. Fatal reactions have been reported with daratumumab-containing products, including daratumumab and hyaluronidase-fihj. Permanently discontinue daratumumab and hyaluronidase-fihj for life-threatening reactions.
- Cardiac Toxicity in Patients With Light Chain (AL) Amyloidosis: Monitor patients with cardiac involvement more frequently for cardiac adverse reactions and administer supportive care as appropriate.
- Neutropenia: Monitor complete blood cell counts periodically during treatment. Monitor patients with neutropenia for signs of infection. Consider withholding daratumumab and hyaluronidase-fihj to allow recovery of neutrophils.
- Thrombocytopenia: Monitor complete blood cell counts periodically during treatment. Consider withholding daratumumab and hyaluronidase-fihj to allow recovery of platelets.
- Embryo-Fetal Toxicity: Can cause fetal harm. Advise pregnant women of the potential risk to a fetus and advise females of reproductive potential to use effective contraception.
- Interference With Cross-Matching and Red Blood Cell Antibody Screening: Daratumumab binds to CD38 on red blood cells (RBCs) and results in a positive Indirect Antiglobulin Test (Indirect Coombs test), which may persist for up to 6 months after the last daratumumab administration. Type and screen patients prior to starting treatment. Inform blood banks that a patient has received daratumumab and hyaluronidase-fihj.
- Interference With Determination of Complete Response: Daratumumab can interfere with the determination of complete response and of disease progression in some patients with IgG kappa myeloma protein.
ADVERSE REACTIONS
POMALYST:
The most common adverse reactions for POMALYST (≥30%) included fatigue and asthenia, neutropenia, anemia, constipation, nausea, diarrhea, dyspnea, upper-respiratory tract infections, back pain, and pyrexia.
In the phase III trial, nearly all patients treated with POMALYST + low-dose dex experienced at least one adverse reaction (99%). Adverse reactions (≥15% in the POMALYST + low-dose dex arm and ≥2% higher than control) included neutropenia (51%), fatigue and asthenia (47%), upper respiratory tract infection (31%), thrombocytopenia (30%), pyrexia (27%), dyspnea (25%), diarrhea (22%), constipation (22%), back pain (20%), cough (20%), pneumonia (19%), bone pain (18%), edema peripheral (17%), peripheral neuropathy (17%), muscle spasms (15%), and nausea (15%). Grade 3 or 4 adverse reactions (≥15% in the POMALYST + low-dose dex arm and ≥1% higher than control) included neutropenia (48%), thrombocytopenia (22%), and pneumonia (16%).
ADVERSE REACTIONS FOR POMALYST + dexamethasone + daratumumab
The most common adverse reactions (≥20%) included neutropenia (95%), lymphopenia (94%), thrombocytopenia (75%), anemia (57%), infusion reactions (50%), fatigue (50%), upper respiratory tract infection (50%), cough (43%), diarrhea (38%), constipation (33%), dyspnea (33%), nausea (30%), muscle spasms (26%), pyrexia (25%), back pain (25%), insomnia (23%), arthralgia (22%), vomiting (21%), dizziness (21%), and chills (20%). Grade 3 or 4 hematology laboratory abnormalities included: neutropenia (82%), lymphopenia (71%), anemia (30%), and thrombocytopenia (20%).
ADVERSE REACTIONS FOR POMALYST + dexamethasone + dara SC
The most common adverse reactions (≥20%) included fatigue (46%), pneumonia (38%), upper respiratory tract infection (36%), and diarrhea (22%). Grade 3 or 4 hematology laboratory abnormalities included: decreased neutrophils (84%), decreased leukocytes (64%), decreased lymphocytes (59%), decreased platelets (19%), and decreased hemoglobin (16%).
DRUG INTERACTIONS FOR POMALYST
Avoid concomitant use of POMALYST with strong inhibitors of CYP1A2. If concomitant use of a strong CYP1A2 inhibitor is unavoidable, reduce POMALYST dose to 2 mg.
USE IN SPECIFIC POPULATIONS FOR POMALYST
- Pregnancy: See Boxed WARNINGS for POMALYST. If pregnancy does occur during treatment, immediately discontinue the drug and refer patient to an obstetrician/gynecologist experienced in reproductive toxicity for further evaluation and counseling. There is a POMALYST pregnancy exposure registry that monitors pregnancy outcomes in females exposed to POMALYST during pregnancy as well as female partners of male patients who are exposed to POMALYST. This registry is also used to understand the root cause for the pregnancy. Report any suspected fetal exposure to POMALYST to the FDA via the MedWatch program at 1-800-FDA-1088 and also to the REMS Call Center at 1-888-423-5436.
- Lactation: There is no information regarding the presence of pomalidomide in human milk, the effects of POMALYST on the breastfed child, or the effects of POMALYST on milk production. Pomalidomide was excreted in the milk of lactating rats. Because many drugs are excreted in human milk and because of the potential for adverse reactions in a breastfed child from POMALYST, advise women not to breastfeed during treatment with POMALYST.
- Pediatric Use: Safety and effectiveness have not been established in pediatric patients.
- Geriatric Use: No dosage adjustment is required for POMALYST based on age. Patients >65 years of age were more likely than patients ≤65 years of age to experience pneumonia.
- Renal Impairment: For patients with severe renal impairment requiring dialysis, reduce POMALYST dosage to 3 mg orally daily. Take dose of POMALYST following hemodialysis on hemodialysis days.
- Hepatic Impairment: For patients with mild to moderate hepatic impairment, reduce POMALYST dosage to 3 mg orally daily and to 2 mg orally daily in patients with severe hepatic impairment.
- Smoking Tobacco: Advise patients that smoking may reduce the efficacy of POMALYST. Cigarette smoking reduces pomalidomide AUC due to CYP1A2 induction.
Please see full Prescribing Information for POMALYST, including Boxed WARNINGS.
Information about DPd and Pd + dara SC does not appear in the POMALYST Prescribing Information (PI). Please see the daratumumab and dara SC full PIs for a complete discussion of Important Safety Information at www.darzalexhcp.com/iv and www.darzalexhcp.com/faspro, respectively.